Pneumococcal Disease And Vaccines: The Numbers’ Game

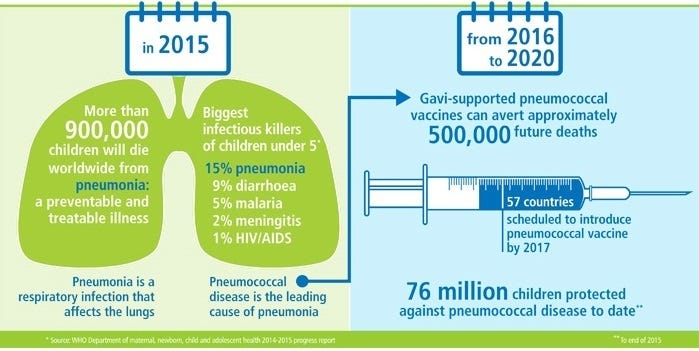
Diseases caused by Streptococcus pneumonia include pneumonia, meningitis (swelling of the membranes covering the brain and spinal cord) and bacteremia (bacteria in the blood). The World Health Organization (WHO) estimates that globally S. pneumoniae kills close to 500,000 children less than 5 years old annually, with most of the deaths occurring in developing countries.

Currently, a 10-valent polysaccharide-protein conjugate vaccine and a 13-valent polysaccharide-protein conjugate vaccine are marketed internationally. Both vaccines are considered safe and effective against vaccine serotypes but do not protect against all 90 plus pneumococcal serotypes. Deployment of pneumococcal conjugate vaccines (PCV) has had clear public health benefits: the incidence of invasive pneumococcal disease has been reduced not only in vaccinated children, but also in elderly adults, who benefit from herd immunity; hospital admissions for community-acquired pneumonia among children less than two years old have been reduced, and the incidence of acute otitis media in healthy vaccinated children has decreased. In 2007, the WHO recommended the use of pneumococcal conjugate vaccines in all countries.
Catch Up Game
An impediment to the accomplishments of PCV is that the vaccines cover just the most common pneumococcal serotypes, giving way to the expansion of and rise in antibiotic resistance of other types (not included in the vaccine). A study in Euro surveillance analysis of S pneumonia isolates collected by the surveillance programs of the British Society for Antimicrobial Chemotherapy (BSAC) and Public Health England (PHE) has found that serotype 15A is becoming increasingly prevalent. Serotype 15A is of particular interest since multidrug-resistant isolates belonging to this serotype have been reported as far apart as Australia, East Asia, Italy, North America and Norway. S pneumoniae 15A is not covered by the 13-valent pneumococcal conjugate vaccine. 15A is one of the more than 90 pneumococcal serotypes not included in the 13-valent vaccine. The rise of 15A means that conjugate vaccines will face an ongoing game of catch-up whereby vaccine manufacturers increase the quantity of serotypes included in the vaccine to cover serotypes that were once not prevalent.
The More the Merrier
Vaccine research is taking place to find a vaccine that is more affordable and offers broad protection across all pneumococcal serotypes. The broader protection is particularly relevant for low-income countries where there is a broader spectrum of serotypes (not in the vaccine) that cause disease. Current manufacturers of the pneumo vaccines have invested a lot to support Gavi in meeting country demand and are developing multi-dose vials that reduce cold chain requirements and allow more children to be vaccinated. Most of these efforts are preclinical and are designed to develop vaccines similar to the currently licensed PCVs Prevenar 13 and Synflorix at a lower cost. Not to be left out, US-based Merck and Co. and France-based Sanofi have also joined the race to develop new PCVs, which are in various stages of clinical development. The most exceptional candidates are 10-to 13-valent PCVs being created by the Serum Institute of India, Ltd., SK Chemical Co., and Panacea Biotech, Ltd. Competition from new companies will definitely bring down prices overall. Nonetheless, because of the complexities of building up a technologically advanced vaccine covering an expansive number of strains of pneumococcal disease, we may only see these new vaccines in 2018 or later.
GlaxoSmithKline made a commitment to provide the pneumococcal vaccine at the lowest ever price at US$3.05 for doses being provided from 2017, a reduction of 10% from the current price of $3.40. The price will be available through the Advance Market Commitment (AMC) to all Gavi countries as well as for countries using the vaccine when they transition from Gavi support and will continue at this price for ten years after the transition. The AMC, designed to stimulate the development and manufacture of vaccines for low-income countries. Donors commit money to guarantee the price of vaccines once they are developed, giving that they meet particular and strict, per-agreed criteria on adequacy, cost, availability, and that developing countries demand them. Pricing of vaccines remain a contentious topic. In October last year, Doctors Without Borders turned down free pneumonia vaccines saying that pharmaceutical companies use donations as a way to make others “pay up.” Companies use this to justify why prices remain high for others. Pfizer has also lowered the price of its pneumococcal vaccine to non-governmental organizations that supply developing countries. The company will sell the newest version of its Prevnar 13 vaccine for $3.10 a dose, the same price that Gavi has paid since 2015.
A Game-changer?

While pneumococcal conjugate vaccines are highly effective, they are extremely complex and generally exorbitant vaccines to make. Pneumococcal protein vaccines, on the contrary, are more efficient and less expensive to manufacture and have the potential to provide broad coverage across all serotypes and avoid the problems of serotype replacement by directly targeting proteins highly preserved among many pneumococcal serotypes. PATH and Boston Children’s Hospital are working on the development of an inactivated, pneumococcal whole cell vaccine candidate (PATH-wSP), that could provide broad protection and be relatively cheap to produce and easy to administer.
Although pneumococcal vaccination coverage is increasing globally, it is nowhere near optimal vaccination rates. A vaccine is only useful if administered. A vaccine not given is a health risk ignored and — for the more vulnerable members of society — a hospitalization or death that could have been prevented.
Originally published at The Huffington Post.
Dr Melvin Sanicas, vaccinologist and public health physician is a regional medical expert at Sanofi Pasteur, a consultant for the World Health Organization, and an agenda contributor for the World Economic Forum. He was a Global Health Fellow and Program Officer at the Bill & Melinda Gates Foundation where he managed the Collaboration for TB Vaccine Discovery. He is a partner at the Brighton Collaboration, a fellow of the Royal Society of Tropical Medicine and Hygiene, and a fellow of the Royal Society for Public Health.